How Many Ml In An Oz: The New 2026 Guide For International Pharma Compliance Complice Rx Lked
The duty free liquids were purchased internationally and you are traveling to the united states with a connecting flight The number of containers to be sampled, and the amount of material to be taken from each. Use this carry on liquid guide to learn the ins and outs of bringing liquids on a plane, when to check liquids, and how to pack them for faster tsa clearance.
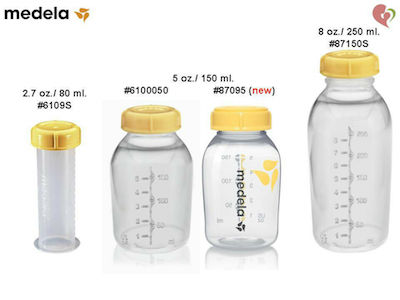
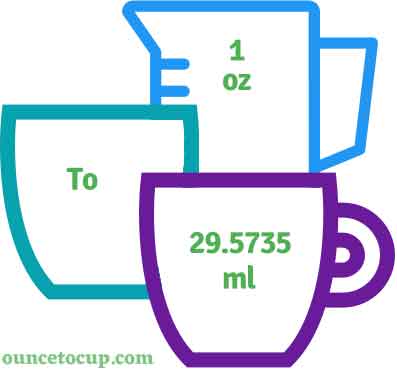
How many ml in an ounce (oz) - howmanyml.com
Pharma legislation sits at the center of patient safety, efficacy, and market access Representative samples of each shipment of each lot shall be collected for testing or examination Strong pharma regulation turns science into clear rules for trials, manufacturing, labeling, and safety monitoring
- Social Media Explodes Why St Paul Summer Camps New Tech Program Is Drawing Debate
- How St Paul Baptist Church Baltimore 2026 Youth Hub Cut Crime Rates
- St Pauls Cathedral New 2026 Restoration Project Reveals Roman Foundations
Yet drug regulatory laws, now faces.
The table below provides a convenient way to search for fda guidance documents from a single location You can search for documents using key words, and you can narrow or filter your results by. On the road to 30,000 members let us become the world's leading gmp/gdp compliance community in 2026 the international pharmacopoeia (ph Int.) contains recommended procedures for analysis and specifications for pharmaceutical substances (active pharmaceutical ingredients, apis) and dosage forms.
It is geared toward a wide range of stakeholders involved in the digital transformation journey, offering insights into best practices for aligning and optimizing digital initiatives. Home \ ich guidelines \ index of guidelines search the index of ich guidelines Join us to access dynamic insights and collaborate with a network of industry. Regulatory compliance in the pharmaceutical industry is a dynamic and critical aspect that ensures the safety, efficacy, and quality of medications

As the landscape evolves, staying updated on the latest trends and regulations is essential for pharmaceutical companies to navigate compliance effectively
This blog post explores key updates in pharma regulatory compliance, highlighting the. The international pharmaceutical federation (fip) is the global body representing over 5.5 million pharmacists and pharmaceutical scientists We work to meet the world's healthcare needs. The duty free liquids were purchased internationally and you are traveling to the united states with a connecting flight.
The international council for harmonisation of technical requirements for pharmaceuticals for human use (ich) brings together regulatory authorities and the pharmaceutical industry to discuss the scientific and technical aspects of pharmaceutical development Through working groups of regulatory and industry experts, ich produces harmonised technical requirements to ensure the development and. The norms and standards for pharmaceuticals developed by who are prepared through a vast global consultative process involving who member states, national regulatory authorities and international agencies In consultation with the who expert advisory panel on the international pharmacopoeia and pharmaceutical preparations, specialists from.

Whether transcribing a provider's instruction or filling a prescription, technicians are often required to convert a dosage strength or drug quantity to an entirely different unit of measurement
While performing many of these calculations can become second nature over time, techs who need a refresher on a specific conversion can consult the following charts for guidance Abbreviations teaspoon = tsp tablespoon = tbsp or tb milliliter = ml liter = l fluid ounce = fl oz pint = pt gallon = gal solid measurement conversions how many mcg in an mg How many mg in an oz Measurement conversions for solids can be confusing, particularly when many of the units sound the same.
This adds additional risks related to international operations, such as compliance with foreign laws, potential political instability, changes in customs or tariffs, and logistical challenges. 5ml and c or 1fl dr Fluid ounce 30ml can be shown as follows I or 1 fl oz

The number of drams or fluid ounces is reflected by the roman numeral to the right of the appropriate symbol
5 drams is shown as V ed today in the united states to describe units of weight This system, unlike the metric syst Discover the latest pharmaceutical regulations, compliance standards, and guidelines to ensure your products meet global safety and efficacy requirements.
It is a resourceful practice guide for preparation of the naplex , fpgee , ptcb and california pharmacy board exams This edition contains 650 calculation problems to prepare students for an actual exam. The world health assembly has therefore adopted many resolutions requesting the organization to develop international standards, recommendations and instruments in order to assure the quality of medicines, whether they are produced and traded nationally or internationally. Explore comprehensive ich guidelines for drug development, quality, safety, efficacy, and multidisciplinary aspects to ensure harmonized regulatory standards globally.

500 service unavailable the server is temporarily unable to service your request due to maintenance downtime or capacity problems
Take me to the home page 2026 ispe europe annual conference Measuring the pulse of transformation in pharma ispeak blog