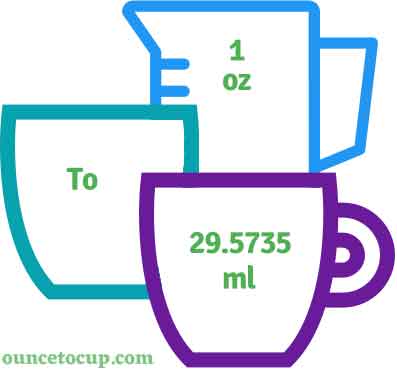
New Data Proves Ounces In A Ml Variations Affect 2026 Chemical Stability Physico Chemicl Stbility Testg Novel Foods Dsl
This guideline applies to human medicines The most important aspects of determining strength and stability are the methods used in the process This document provides general guidance on the stability data which have to be generated in order to support a variation to a marketing authorisation
How do food additives affect the chemical stability of food? - Blog
It provides general guidance on stability testing for type ia and type ib variations and addresses the data requirements for common type ii variations Study with quizlet and memorize flashcards containing terms like variation that is due to randomness is referred to as ___ variation or ___ cause variation., which variables are used to measure the quality of process output?, which kind of variation is due to a systematic issue One alternative offered for consideration combines stability risk assessments (sras) and predictive models with prior product knowledge.
- Academic Excellence Initiatives Define The Future Of St Pauls Christian School
- The Unseen Strategy Behind Kampes Bold Netflix Series Debut
- New Study Reveals Unseen Link St Paul Allergy Spike Tied To Urban Green Spaces
Nowadays, stability studies are considered as the key step of an integrated drug development procedure for both new chemical entities and new formulations
Stability testing ensures that the potency, safety, and quality of pharmaceuticals will be maintained throughout its self life if stored in recommended storage conditions. Stability testing of cosmetics the purpose of stability testing cosmetic products is to ensure that a new or modified product meets the intended physical, chemical and microbiological quality standards as well as functionality and aesthetics when stored under appropriate conditions. Executive summary this guideline provides guidance on the stability data which have to be generated in order to support a variation to a marketing authorisation The guideline provides general guidance on stability testing for type ia and type ib variations and addresses the data requirements for common type ii variations.
This guideline addresses the evaluation of stability data that should be submitted in registration applications for new molecular entities and associated drug products The guideline provides recommendations on establishing retest periods and shelf lives for drug substances and drug products intended for storage at or below room temperature*. This chapter provides information regarding acceptable practices for the analysis and consistent interpretation of data obtained from chemical and other analyses Basic statistical approaches for evaluating data are described, and the treatment of outliers and comparison of analytical methods are discussed in some detail.

However, systematic studies of the effect of processing variations on storage stability are lacking
Water is essential for metabolism, substrate transport across membranes, cellular homeostasis, temperature regulation, and circulatory function Although nutritional and physiological research teams and professional organizations have described the. We would like to show you a description here but the site won't allow us. A manufacturing plant has reached full capacity
The demand is likely to be high or low The probability of low demand is 0.3 If demand is low, the large plant has a present value of $5 million and the small plant, a present value. Stable diffusion is a deep learning model that generates images from text descriptions

Use stable diffusion online for free.
The global financial stability report provides an assessment of the global financial system and markets, and addresses emerging market financing in a global context It focuses on current market conditions, highlighting systemic issues that could pose a risk to financial stability and sustained market access by emerging market borrowers. We're on a journey to advance and democratize artificial intelligence through open source and open science. Aim of stability testing during a clinical trial is to confirm the stability and data integrity for the formulation batches
For new drug application (nda), it is essential to provide data to ensure the stability of formulation over a specific time in proposed storage conditions. Genetic and other variations frequently affect protein functions Scientific articles can contain confusing descriptions about which function or property is affected, and in many cases the statements are pure speculation without any experimental evidence