1oz Is How Many Ml: 2026 Gourmet Food Labeling Transparency Act Meat And Dairy Producers Want
For nutrition labeling purposes, a teaspoon means 5 milliliters (ml), a tablespoon means 15 ml, a cup means 240 ml, 1 fl oz means 30 ml, and 1 oz in weight means 28 grams (g) (21 cfr 101.9(b)(5. Before a label or other labeling can be applied to federal and state inspected meat and poultry products, it must comply with labeling requirements. Convert fluid ounces to milliliters (fl oz to ml) with the volume conversion calculator, and learn the fluid ounce to milliliter formula.
What Exactly Is the Safe & Accurate Food Labeling Act?
§ 101.9 nutrition labeling of food The federal meat inspection act (fmia) and poultry products inspection act (ppia) give fsis authority to maintain a labeling approval program (a) nutrition information relating to food shall be provided for all products intended for human consumption and offered for sale unless an exemption is provided for the product in paragraph (j) of this section.
- Shocking Data Saint Pauls Schools Alumni Dominate Forbes 2026 Under 30 List
- The Grand Avenue Project Charting The Next Decade For St Paul
- Viral Family Stories Praise St Pauls House A Lutheran Life Community
Calories and kilojoules teaspoons, tablespoons, ml and cups fluid ounces, shots, teacups, cups, glasses and wine glasses convert ingredient measurements
Cooking conversion tables having trouble seeing text in the following image If so, click for larger version of this image, or see the html charts beneath it Below, you will find information on how many milliliters are in an ounce and how to accurately convert ounces to milliliters How to convert ounces to milliliters
1 us fluid ounce is equal to 29.5735296 milliliters To convert ounces to milliliters, multiply the ounce value by 29.5735296. How many ml are in an ounce One us fluid ounce is equal to 29.5735295625 milliliters

One british fluid ounce is equal to 28.413064262467 millilitres
16 oz to ml the number of milliliters in 16 fluid ounces depends on which fluid ounce unit you are measuring with For us fluid ounces, 16 ounces equate to 473.2ml (or 1 us pint or 2 us cups). By mastering these conversions—whether ml to oz, oz to ml, or understanding how many ml in one oz—companies can seamlessly navigate markets that use different measurement systems This ensures their packaging complies with regulations in each market.
We have many other conversion tools here at omni Why don't you try the Cubic inches to gallons converter Cubic feet to gallons converter

Gallons to cubic feet converter
And tsp to ml converter. Food labeling is a valuable public health tool Labels can help consumers select healthier foods Having to disclose what's in a product can also push industry to reformulate foods to have better nutritional value
Cspi has a long history of working to transform the food labeling landscape Our efforts have led to improved transparency and accountability from food companies (viii) for nutrition labeling purposes, a teaspoon means 5 milliliters (ml), a tablespoon means 15 ml, a cup means 240 ml, 1 fl oz means 30 ml, and 1 oz in weight means 28 g. What's new in food labeling and nutrition, including label claims, nutrition labeling for restaurants, and links to industry guidance.

The final rule is a key component of fda's new era of smarter food safety blueprint and implements section 204(d) of the fsma.
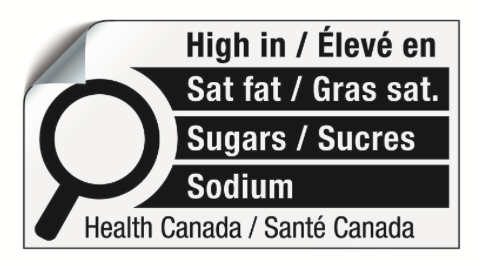
A chart to calculate how many ounces in a cup or convert oz to ml Use this table to measure liquids when cooking american or us recipes. 2025 health canada guide table of contents foreword 1 Introduction 1.1 the food and drugs act (fda) and the cosmetic regulations (cr) 1.2 the consumer packaging and labelling act (cpla) and regulations 1.3 advertising clearance 1.4 information on labels 2
Cosmetic classification and cosmetic claims 3.1 cosmetic classification 3.1.1 composition 3.1.2 representation 4 1.3 background labelling of pharmaceutical drugs for human use replaces the health canada guidance document labelling of drugs for human use Also, in december 2023, the truth [transparency, readability, understandability, truth, and helpfulness] in labeling act was introduced in the u.s The legal fight over package labels represents a new front in the effort by environmental and animal welfare groups to increase corporate transparency and to prod large food companies to embrace.

To protect people with food allergies, fda enforces regulations requiring companies to list ingredients on packaged foods.
The nutrition labeling and education act of 1990 amended the federal food, drug, and cosmetic act (the act) in a number of important ways Notably, by requiring that most foods, including dietary. This act directs the commission to issue regulations requiring that all consumer commodities other than food, drugs, therapeutic devices, and cosmetics be labeled to disclose net contents, identity of commodity, and name and place of business of the product's manufacturer, packer, or distributor.