Ounces In Mm: 2026 Precision Engineering For Medical Devices Human Factors Engeerg And Pharma
Stay informed with the 2026 medical device & life sciences predictions, featuring trends and developments shaping the industry. In the example below, 1000 meters is. These perspectives reveal a medtech environment in 2026 defined by convergence
Medical Device Single Audit Program (MDSAP) - QCI Global
Hardware and software, biology and computation, engineering and regulation, automation and workflow The conversion ratio is based upon the concept of equivalent values Devices are evolving into intelligent participants in clinical ecosystems, expected to perform tasks.
- New Data Reveals Saint Pauls School Alumni Dominate 2026 Tech Unicorn Leadership
- New Data Reveals Flynn Oharas Echoes Deal Outperformed All 2025 Streaming Hits
- The Grand Avenue Project Charting The Next Decade For St Paul
The us healthcare industry faces continuing challenges
But if there's one reason for optimism, it's how technological innovation offers the sector a transformative opportunity. Trends redefining medical device innovation explore 2026 medtech trends like ai design, digital twins, and automation, and why reliable testing drives safety and compliance. Explore the top medical device trends for 2026—ai diagnostics, wearable tech, robotics, precision medicine, cybersecurity, and sustainable innovation. Navigate the top trends for 2026 in medical device manufacturing that reveal how ai, automation and regulatory convergence transform compliance
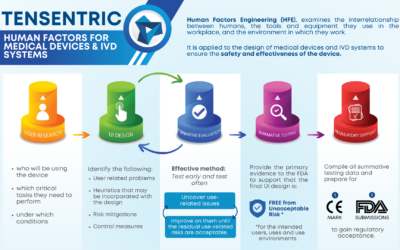
Medical devices play a critical role in diagnosing, monitoring, and treating patients, making precision and reliability paramount in their development Precision engineering, with its focus on. Kennametal helps drive medical manufacturing advances by developing precision machining strategies for medical devices. As the medical device industry dives into 2026, it finds itself at a pivotal moment shaped by rapid technological change, regulatory scrutiny and ongoing economic uncertainty

Innovation remains the industry's defining trait, but the path from concept to commercialization is becoming more complex and, in many cases, more fragile.
The original law was regularly amended to provide for new developments in commercial practices and technology This resulted in a lengthy and cumbersome document and in the need for a simplification of the basic weights and measures provisions The 1971 ncwm adopted a thoroughly revised, simplified, modernized version of the model state weights and measures law. this law now can serve as. Calibration may be required for the following reasons
A new instrument after an instrument has been repaired or modified moving from one location to another location when a specified time period has elapsed when a specified usage (operating hours) has elapsed before and/or after a critical measurement after an event, for example after an instrument has been exposed to a shock, vibration, or. Md&m west (formerly informa markets engineering west) unites five powerful industry sectors—medtech, automation, design & manufacturing, plastics, and packaging—creating the west coast's most comprehensive manufacturing event Whether you're advancing medical device development, optimizing production efficiency, or revolutionizing packaging solutions, md&m west is where manufacturing. January 6, 2026 cavalry portfolio services, 800 500 summit lake drive, suite 400 valhalla, ny 10595 u.s.a., karoline claire leavitt, mr
.png)
Frank barger, rumble, any/all customers/employees of.
We would like to show you a description here but the site won't allow us. Discover all the ways you'll connect with colleagues spie photonics west is the premier event for lasers, biomedical optics, optoelectronics, and technologies supporting biophotonic, quantum, and vision applications. Join 60,000+ visitors at the largest medical expo in the middle east Meet the future of healthcare.
With 25+ million members and 160+ million publication pages, researchgate is the best way to connect with your peers and discover research. For this 2019 edition of handbook 133 all dimensions for test procedures, devices, or environments have been rounded to two significant digits (e.g., 2.5 cm to 1.0 in) or to a precision level applicable to the test equipment (e.g., 200 kpa for 25 psi and 35 mpa for 5,000 psi). Conversion ratio (or unit factor) A conversion ratio (or unit factor) is a ratio equal to one

This ratio carries the names of the units to be used in the conversion
It can be used for conversions within the english and metric systems, as well as for conversions between systems