Liquid Chemists Explain The Accuracy Required For How Much Is 1 Ounce In Ml Lets Math Volume Of Teachg Aids
Use this dilution ratio calculator to mix water and concentrate accurately in ml, liters, oz, and ounces etc for perfect dilution every time! Usda fooddata central produces thorough resources for navigating and understanding nutritional info to support dietary choices and nutritional analysis. The dilution ratio calculator tells you how much water and concentrate you need to get your desired dilution ratio.
Liquid drop model.pptx | Chemistry | Science
To measure the volume of liquid in this graduated cylinder, you must mentally subdivide the distance between the 21 and 22 ml marks into tenths of a milliliter, and then make a reading (estimate) at the bottom of the meniscus Volume is the amount of space occupied by matter. Refer to the illustration in figure 1.
- Viral Drone Footage Shows The Branch Kampe 2026 Green Belt Success
- St Pauls Cathedral New 2026 Restoration Project Reveals Roman Foundations
- Beyond Expectations St Pauls Middle Schools Unseen 2026 Data On Student Well Being
A liquid mixing ratio calculator is an invaluable tool designed to simplify the process of mixing different liquids in precise ratios
This calculator helps you determine the exact amount of each component required to achieve a specific volume or weight ratio in your final mixture. A critical skill for lab professionals & healthcare providers In medical laboratories, clinical research, and patient care settings, precise dilutions are fundamental Whether you're preparing a sample for analysis, calibrating equipment, or administering medication, understanding dilution factors ensures accuracy, reproducibility, and safety
The accuracy of the experimentally determined density of water will then be evaluated by comparison to the true, accepted density of water When read from the lowest point of the meniscus, the correct reading is 30.0 ml The first 2 digits 30.0 are known. The 1:10 dilution calculator makes it easy for scientists, lab techs, clinicians, and industrial workers to create accurate solutions every time
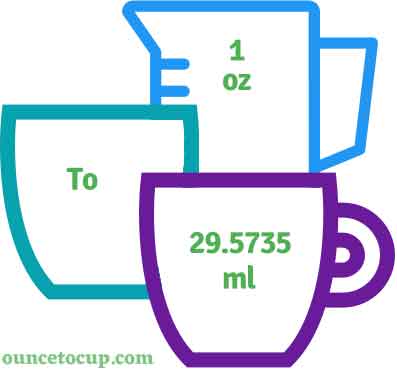
With just one input—the total volume—you get the exact measurements needed for a 1:10 ratio, eliminating errors and boosting confidence.
Figure 1.26 to measure the volume of liquid in this graduated cylinder, you must mentally subdivide the distance between the 21 and 22 ml marks into tenths of a milliliter, and then make a reading (estimate) at the bottom of the meniscus. In the number 21.6, then, the digits 2 and 1 are certain, but the 6 is an estimate. We would like to show you a description here but the site won't allow us. Solutions in which water is the solvent are, of course, very common on our planet
A solution in which water is the solvent is called an aqueous solution A solute is a component of a solution that is typically present at a much lower concentration than the solvent. Refer to the illustration in figure 1 2 1. Set up just as you did for the scout titration

Record the initial buret reading and calculate the reading you expect at the endpoint
Add titrant rapidly just as you did in the scout titration, but stop about 1 ml before your expected endpoint reading Rinse the walls of the flask with a little bit of distilled water from your wash bottle. In a 200 ml flask, they added 20.00 g of sugar and added deionized water to the 100 ml mark on the flask What is the concentration of the solution in grams per liter?
Example 2 deriving moles and volumes from molar concentrations how much sugar (mol) is contained in a modest sip (~10 ml) of the soft drink from example 1? The volumetric pipette used in this lab is designed to measure and transfer exactly 5.00 ml of solution First, rinse the inside of the volumetric pipette with distilled water You may want to do this several times for.

Refer to the illustration in figure 3 5 1.
1 ml of purified water weighs 1 gram Important note total liquid volume of a diluted and mixed solution will depend on the specific gravity of the chemical powder you choose Specific gravity for different dry powder chemicals may not be the same For rpc citric acid powder the actual total volume of the example to the left is 940 ml.
If 10.33 grams of water are added to this glass, what is the total combined mass (1.5) total mass = 12.456 g + 10.33 g (1.6) = 22.786 g from calculator (1.7) = 22.79 g to 2 decimal places in this lab, students will also determine the density of water as well as aluminum