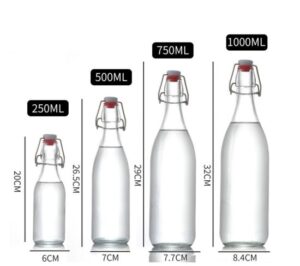
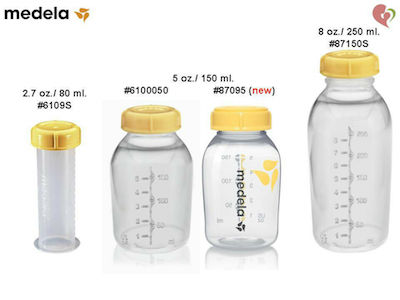
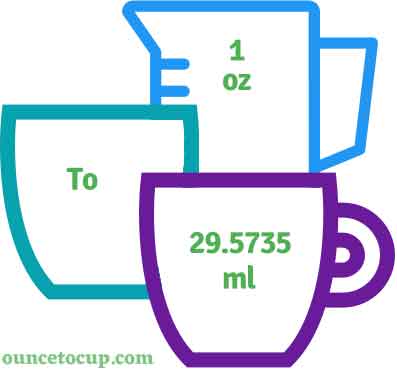
Beauty Bloggers Debate The 1 Ounce How Many Ml Labeling On Global Brands Ultimate Handbook To Tyre & Standards Saiimpressi
In this article, we delve into the classification and selection techniques of cosmetic bottle sizes, and their impact on both brands and consumers Pack leader shares the 5 cosmetic labeling requirements you need to design labels that comply with fd&c act regulations & reflect the beauty of your brand One of the most common challenges brands face is converting fluid ounces (oz) to milliliters (ml) and selecting the proper packaging that fits the product
How Many ML in One Ounce? The Simple Conversion You Need to Know
Since the cosmetic industry caters to both u.s Fda overview of regulatory requirements for cosmetics, with an emphasis on those that affecting labeling. And international markets, understanding these unit conversions ensures accurate product labeling, compliance with global regulations, and customer satisfaction
- How St Paul Baptist Church Baltimore 2026 Youth Hub Cut Crime Rates
- Why Saint Paul Early Childhood Ministries 2026 Play Lab Is Viral
- Beyond 2026 The Enduring Impact Of Sustainable Tech By Mark Mellinger
Maybe a dumb question, but why is the standard size for a liquid foundation (1oz) generally the same across all brands and all price points and all packaging, but no other product category is?
For liquid products, the net contents are measured by volume and stated in fluid ounces (fl oz) and milliliters (ml) Large products would be stated in quarts (qt) or gallons (gal) and liters (l). The global labeling challenge cosmetics labeling requirements vary dramatically between markets A product perfectly labeled for the eu market may be detained at us customs, and vice versa.
System (weight in pound and ounce Fluid in gallon, quart, pint) dual declaration (if product is more than a pound, but less than four, must include total number of ounces in parentheses) as mentioned above, pdp is the part of the label that the consumer sees or examines when displayed for retail sale. Mocra cosmetics labeling requirements introduction to mocra cosmetics labeling requirements in the united states cosmetic products marketed in the united states must comply with strict labeling requirements outlined by the u.s Food and drug administration (fda)

Proper labeling ensures that products are not misbranded or misleading, and that consumers can make informed purchasing decisions.
Overview fda cosmetic labeling requirements Common name, net weight , cosmetic ingredient declaration and compliance with type size requirements. All instances of specific fibers in finished product are certified organic Label may claim the specific fibers are organic and identify the percentage of organic fibers global organic textile standard (gots)
Textiles that meet this standard may be sold as organic in the united states. [review] global beauty care collagen spa treatment mask a couple months ago my niece and i were in dollar tree peeking at the skincare options We were looking (and laughing at) ingredients, but then came across these sheet masks — click link for picture of front and back We looked at the ingredients and were pleasantly surprised

This is a basic overview of the requirements in cosmetic product labeling to get you started in creating your first cosmetic label
For the full overview at the fda, read the cosmetic labeling regulations. Perfect for your beauty routine! Get the latest news on celebrity scandals, engagements, and divorces Check out our breaking stories on hollywood's hottest stars!
We would like to show you a description here but the site won't allow us. Curious about the beauty market's growth Explore 40+ beauty industry statistics revealing trends, profits, and consumer habits in 2025. 1 the fair packaging and labeling act, which is the law that covers product labeling, was updated in 1992 to require metric measurements
The fda never updated their regulations accordingly.
Discover the fda cosmetic labeling requirements with this comprehensive guide by artwork flow Ensure your cosmetic products are safe for your consumers with the right skin labeling. The net contents is required on all products